Corrosion is the damage or deterioration of materials or their properties caused by the action of the environment. Most corrosion occurs in the atmospheric environment, which contains corrosive components and corrosive factors such as oxygen, humidity, temperature changes and contaminants.
Salt spray corrosion is a common and most destructive form of atmospheric corrosion. Salt spray corrosion on the surface of metal materials is caused by the chloride ions contained in the metal surface penetrating through the oxidation layer and protective layer and the internal metal electrochemical reaction. At the same time, the chloride ion contains a certain amount of hydration energy, which is easy to be adsorbed in the metal surface pores and cracks and to replace the oxygen in the oxide layer, thus transforming the insoluble oxide into soluble chloride and the passivated state surface into an active surface.
Salt corrosion protection spray test is an environmental test that mainly uses the artificial simulated salt spray environmental conditions created by salt spray test equipment to assess the corrosion resistance of products or metal materials. It is divided into two types of tests: natural environment exposure test, and artificially accelerated simulation salt spray environmental test.
In an artificial simulation salt spray environment test, the salt spray test chamber with certain volume of space is used, and salt spray environment is generated by using artificial methods in its volume of space, so as to assess the performance and quality of salt spray corrosion resistance of products.
The salt concentration of chloride in the salt spray environment can be several times or dozens of times the salt spray content in the ordinary natural environment, thus greatly increasing the corrosion rate and greatly reducing the time to obtain the results. For example, it may take one year to corrode when testing a product sample in the natural exposure environment, while you can get similar test results just after 24 hours in the artificial simulation salt spray environment.
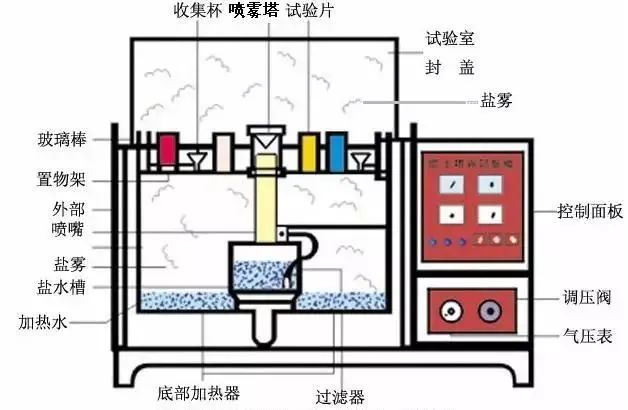
Laboratory simulated salt spray can be divided into four categories.
(1) The neutral salt spray test (NSS test) is the earliest and most widely used accelerated corrosion test method. It uses 5% sodium chloride salt water solution, with the pH value adjusted to a neutral range (6.5~7.2) as a spray solution. The test temperature is 35 ℃, and the required sedimentation rate of salt spray is 1~2ml/80cm/h.
(2) Acetic acid salt spray test (ASS test) is developed on the basis of neutral salt spray test. It is in 5% sodium chloride solution with some glacial acetic acid, so that the solution PH value is reduced to about 3, the solution becomes acidic, and the salt spray formed finally becomes acidic from neutral salt spray. Its corrosion rate is about 3 times faster than the NSS test.
(3) The copper salt accelerated acetate spray test (CASS test) is a newly developed foreign rapid salt spray corrosion test. The test temperature is 50 ℃. A small amount of copper salt-copper chloride is added to the salt solution to strongly induce corrosion. Its corrosion rate is about 8 times that of the NSS test.
(4) Alternating salt spray test is a comprehensive salt spray test, which is actually a neutral salt spray test plus constant humidity and heat test. It is mainly used for the cavity-type product. Through the penetration of the tidal environment, the salt spray corrosion is produced not only on the surface of but also inside the product. The product is alternately converted between the salt spray and humidity and heat environment, and then the electrical and mechanical properties of the product should be assessed for any change.
Result determination
The test result of the salt spray test is generally given in qualitative form rather than quantitative form. There are four specific methods of determination.
(1) Rating determination method.
In this method, divide the ratio of the corrosion area and the total area into several levels, and determine a certain level as the qualified basis for determination. This method is suitable for evaluation of flat samples.
(2) Weighing determination method.
Through weighing the weight of the sample before and after the corrosion test, calculate the weight lost due to corrosion, and judge the spray corrosion protection quality of the sample. This method is particularly suitable for the assessment of certain metal corrosion resistance quality.
(3) Corrosion data statistical analysis method.
This method provides the confidence level of designing corrosion tests, analyzing corrosion data, and determining corrosion data, which is mainly used for analysis and statistics of corrosion, rather than specifically for product quality determination.
Salt spray test of stainless steel
Since invented in the early twentieth century, salt spray test has been highly favored by users of corrosion-resistant materials due to its advantages including reduced time and cost, able to test a variety of materials, and providing simple and clear results.
In practice, the salt spray test of stainless steel is the most widely known, and practitioners must be familiar with how many hours the salt spray test can last for this material.
Material dealers will often extend the salt spray test time of stainless steel with methods such as passivation or increasing the surface polish grade. However, the most critical determining factor is the composition of the stainless steel itself, i.e. the content of chromium, molybdenum and nickel.
The higher the content of both chromium and molybdenum, the greater the corrosion resistance required for pitting and crevice corrosion to begin to appear. This corrosion resistance is expressed by the so-called pitting resistance equivalent (PRE) value: PRE = %Cr + 3.3 x %Mo.
While nickel does not increase the resistance of steel to pitting and crevice corrosion, it can be effective in slowing down the corrosion rate once the corrosion process has begun. Therefore, austenitic stainless steels containing nickel tend to perform much better in salt spray tests and rust much less than low nickel ferritic stainless steels with similar pitting resistance equivalents.
It should be noted that the salt corrosion protection spray test has major drawbacks when testing the performance of stainless steel. The chloride content of the salt spray in the salt spray test is extremely high and far exceeds the real environment, so stainless steels that can resist corrosion in actual applications with very low chloride content will also corrode in the salt spray test.
The salt spray test changes the corrosion behavior of stainless steel, which can be considered neither an accelerated test nor a simulation experiment. The results are one-sided and do not have an equivalent relationship with the actual performance of the stainless steel that is finally put into use.
So you can use the salt spray test to compare the corrosion resistance of different types of stainless steel, but this test is only capable of rating the material. When selecting a specific stainless steel material, the salt spray test alone usually does not provide sufficient information because the connection between the test conditions and the actual application environment is seldom known.
In addition, different categories of steel can not be compared with each other, because the two materials used in the test have different corrosion mechanisms,so the test results and the relevance of the final actual use of the environment is not the same.
Post time: Jul-08-2022

